Home » Publications » Page 5
Carsten Carlberg, Marianna Raczyk, Natalia Zawrotna
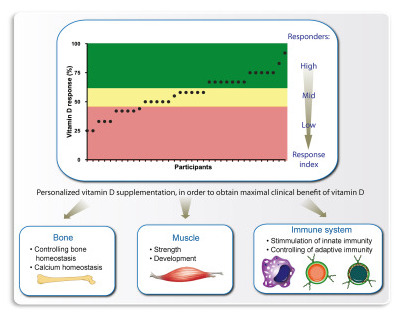
Vitamin D: A master example of nutrigenomics
Redox Biology, Volume 62, 2023
Nutrigenomics attempts to characterize and integrate the relation between dietary molecules and gene expression on a genome-wide level. One of the biologically active nutritional compounds is vitamin D3, which activates via its metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) the nuclear receptor VDR (vitamin D receptor). Vitamin D3 can be synthesized endogenously in our skin, but since we spend long times indoors and often live at higher latitudes where for many winter months UV-B radiation is too low, it became a true vitamin. The ligand-inducible transcription factor VDR is expressed in the majority of human tissues and cell types, where it modulates the epigenome at thousands of genomic sites. In a tissue-specific fashion this results in the up- and downregulation of primary vitamin D target genes, some of which are involved in attenuating oxidative stress. Vitamin D affects a wide range of physiological functions including the control of metabolism, bone formation and immunity. In this review, we will discuss how the epigenome- and transcriptome-wide effects of 1,25(OH)2D3 and its receptor VDR serve as a master example in nutrigenomics. In this context, we will outline the basis of a mechanistic understanding for personalized nutrition with vitamin D3.
https://doi.org/10.1016/j.redox.2023.102695
View full text

Marcus Schmidt & Marianna Raczyk
FODMAP reduction strategies for nutritionally valuable baking products: current state and future challenges
Critical Reviews in Food Science and Nutrition
Fermentable oligo-, di- and monosaccharides and polyols (FODMAP) comprise several previously unrelated carbohydrates, such as fructans, fructo-oligosaccharides, galacto-oligosaccharides, fructose (in excess of glucose), mannitol and sorbitol, and among others. For many patients with gastro-intestinal disorders, such as irritable bowel syndrome, the ingestion of FODMAP triggers symptoms and causes discomfort. Among the main contributors to the dietary FODMAP intake are baking products, in particular bread as a major global staple food. This is primarily due to the fructan content of the cereal flours, but also process induced accumulation of FODMAP is possible. To provide low-FODMAP baking products, researchers have investigated various approaches, such as bio-process reduction by yeast, lactic acid bacteria, germination of the raw material or the use of exogenous enzymes. In addition, the selection of appropriate ingredients, which are either naturally or after pretreatment suitable for low-FODMAP products, is discussed. The sensory and nutritional quality of low-FODMAP baking products is another issue, that is addressed, with particular focus on providing sufficient dietary fiber intake. Based on this information, the current state of low-FODMAP baking and future research necessities, to establish practical strategies for low-FODMAP products, are evaluated in this article.
https://doi.org/10.1080/10408398.2023.2195026
View full text

Carsten Carlberg
Nutrigenomics in the context of evolution
Redox Biology Volume 62, June 2023, 102656
Nutrigenomics describes the interaction between nutrients and our genome. Since the origin of our species most of these nutrient-gene communication pathways have not changed. However, our genome experienced over the past 50,000 years a number of evolutionary pressures, which are based on the migration to new environments concerning geography and climate, the transition from hunter-gatherers to farmers including the zoonotic transfer of many pathogenic microbes and the rather recent change of societies to a preferentially sedentary lifestyle and the dominance of Western diet. Human populations responded to these challenges not only by specific anthropometric adaptations, such as skin color and body stature, but also through diversity in dietary intake and different resistance to complex diseases like the metabolic syndrome, cancer and immune disorders. The genetic basis of this adaptation process has been investigated by whole genome genotyping and sequencing including that of DNA extracted from ancient bones. In addition to genomic changes, also the programming of epigenomes in pre- and postnatal phases of life has an important contribution to the response to environmental changes. Thus, insight into the variation of our (epi)genome in the context of our individual’s risk for developing complex diseases, helps to understand the evolutionary basis how and why we become ill. This review will discuss the relation of diet, modern environment and our (epi)genome including aspects of redox biology. This has numerous implications for the interpretation of the risks for disease and their prevention.
https://doi.org/10.1016/j.redox.2023.102656
View full text

Paweł Płudowski, Beata Kos-Kudła, Mieczysław Walczak, Andrzej Fal, Dorota Zozulińska-Ziółkiewicz, Piotr Sieroszewski, Jarosław Peregud-Pogorzelski, Ryszard Lauterbach, Tomasz Targowski, Andrzej Lewiński, Robert Spaczyński, Mirosław Wielgoś, Jarosław Pinkas, Teresa Jackowska, Ewa Helwich, Artur Mazur, Marek Ruchała, Arkadiusz Zygmunt, Mieczysław Szalecki, Artur Bossowski, Justyna Czech-Kowalska, Marek Wójcik, Beata Pyrzak , Michał A. Zmijewski, Paweł Abramowicz, Jerzy Konstantynowicz, Ewa Marcinowska-Suchowierska, Andrius Bleizgys, Spirydon N. Karras, William B. Grant, Carsten Carlberg, Stefan Pilz, Michael F. Holick and Waldemar Misiorowski
Guidelines for Preventing and Treating Vitamin D Deficiency
Nutrients 2023, 15(3), 695;
All epidemiological studies suggest that vitamin D deficiency is prevalent among the Polish general population. Since vitamin D deficiency was shown to be among the risk factors for many diseases and for all-cause mortality, concern about this problem led us to update the previous Polish recommendations. Methods: After reviewing the epidemiological evidence, casecontrol studies and randomized control trials (RCTs), a Polish multidisciplinary group formulated questions on the recommendations for prophylaxis and treatment of vitamin D deficiency both for the general population and for the risk groups of patients. The scientific evidence of pleiotropic effects of vitamin D as well as the results of panelists’ voting were reviewed and discussed. Thirty-four authors representing different areas of expertise prepared position statements. The consensus group, representing eight Polish/international medical societies and eight national specialist consultants, prepared the final Polish recommendations. Results: Based on networking discussions, the ranges of total serum 25-hydroxyvitamin D concentration indicating vitamin D deficiency [<20 ng/mL (<50 nmol/L)], suboptimal status [20–30 ng/mL (50–75 nmol/L)], and optimal concentration [30–50 ng/mL (75–125 nmol/L)] were confirmed. Practical guidelines for cholecalciferol (vitamin D3) as
the first choice for prophylaxis and treatment of vitamin D deficiency were developed. Calcifediol dosing as the second choice for preventing and treating vitamin D deficiency was introduced. Conclusions: Improving the vitamin D status of the general population and treatment of risk groups of patients must be again announced as healthcare policy to reduce a risk of spectrum of diseases. This paper offers consensus statements on prophylaxis and treatment strategies for vitamin D deficiency in Poland.
Guidelines for Preventing and Treating Vitamin D Deficiency [PDF]
View full text







