Emilia Gospodarska, Ranjini Ghosh Dastidar, Julia Jaroslawska, Maciej Rybiński,Marianna Raczyk, Kornelia Tokarczyk-Malesa, Jerzy Romaszko2 & Carsten Carlberg
Transcriptomic profiling of immune modulation induced by vitamin D3 in the VitDPAS and VitDHiD cohort studies
Scientific Reports
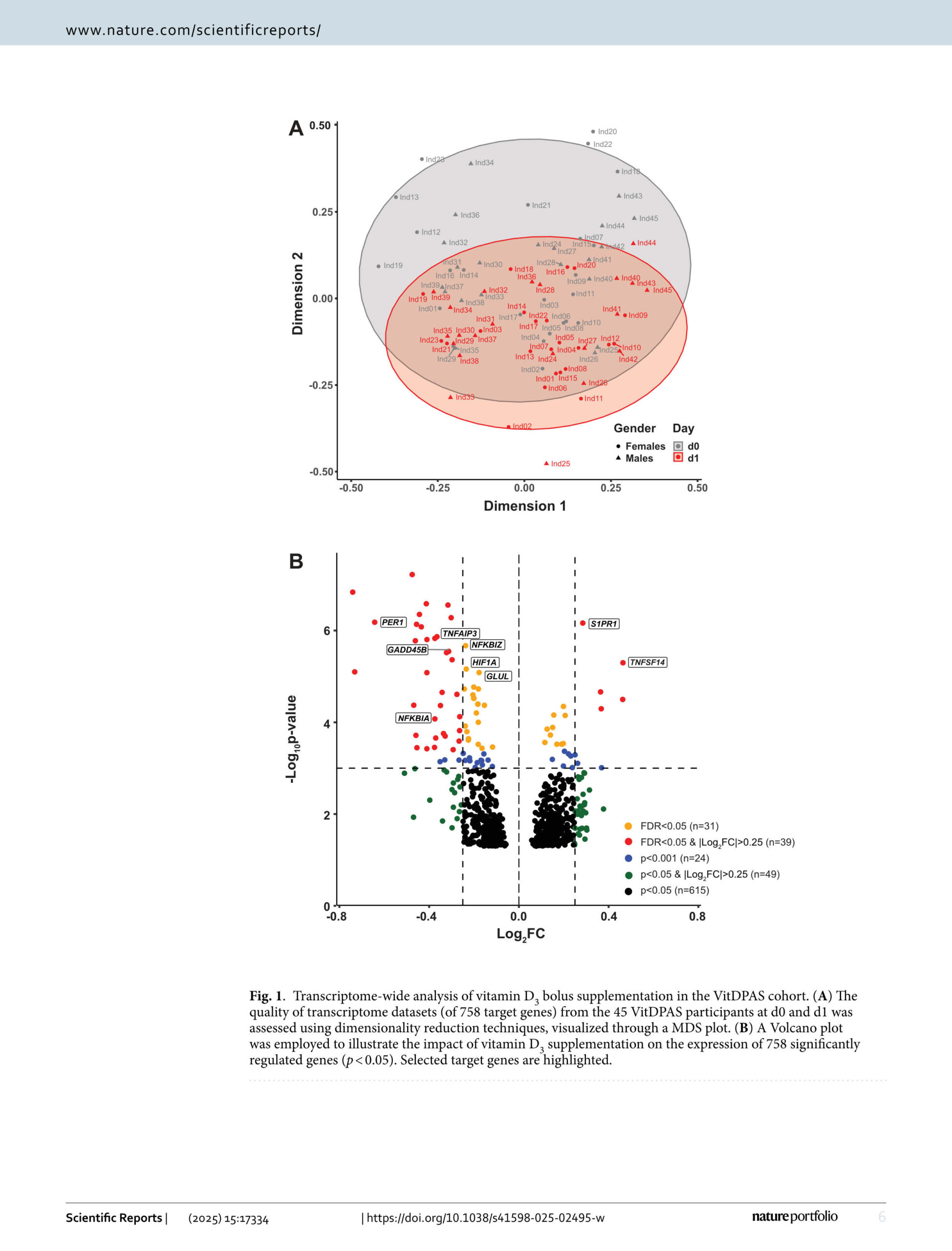
The VitDPAS study (NCT06104111) was designed as a medical experiment to assess the in vivo effects of vitamin D on immune responses. This study enrolled 45 healthy individuals from Olsztyn, Poland, who received a body weight-adjusted bolus dose of vitamin D3 (1,000 IU/kg). Transcriptome-wide differential gene expression analysis of peripheral blood mononuclear cells, collected before and 24 h after supplementation, identified 758 significantly responsive genes (p < 0.05). By correlating individual gene expression changes with alterations in vitamin D status, participants were categorized into three response groups: 17 high responders, 19 mid responders, and 9 low responders. A comparative analysis with the VitDHiD study (NCT03537027), conducted on a Finnish cohort of 25 healthy participants, revealed 232 overlapping target genes, enabling an integrated assessment of vitamin D responsiveness across all 70 individuals. Applying a more stringent statistical threshold (false discovery rate < 0.05) highlighted 26 shared target genes, demonstrating a consistent in vivo response to vitamin D3 across both cohorts. The modulation of inflammatory processes, mediated primarily via tumor necrosis factor and nuclear factor κB signaling pathways, emerged as a shared effect, highlightening the immunomodulatory potential of vitamin D as a key function of the vitamin in healthy individuals.
View full text